What is Up Catalyst and How Does It Enhance Chemical Reactions
In the realm of chemical engineering, the optimization of chemical reactions is paramount for enhancing efficiency and productivity. Recent studies have shown that the implementation of catalysts can significantly accelerate reaction rates, reduce energy consumption, and improve product yields. Among various types of catalysts, "up catalyst" has emerged as a promising tool to enhance chemical reactions, offering innovative pathways for industries ranging from pharmaceuticals to energy production. According to a report by the International Journal of Chemical Engineering, catalysts can increase the reaction rate by up to 100 times, demonstrating the critical role they play in streamlining industrial processes.
The concept of up catalysts is rooted in their unique ability to lower activation energy, thus facilitating faster and more effective chemical transformations. Research indicates that by utilizing up catalysts, industries could save substantial amounts in operational costs while simultaneously decreasing their environmental footprint. For instance, a recent market analysis highlighted that the global catalyst market is expected to reach $35 billion by 2025, driven largely by advancements in catalytic technologies like up catalysts that enhance reaction conditions and improve selectivity. As industries continue to tackle sustainability and efficiency challenges, the significance of up catalysts will undoubtedly grow, making them an essential focus for further innovation and investment.

What is Up Catalyst: Definition and Overview
An up catalyst is a substance that accelerates the rate of chemical reactions without undergoing any permanent change itself. In essence, it facilitates the transformation of reactants into products, lowering the activation energy required for the reaction to occur. This unique property allows reactions that might otherwise be slow or inefficient to proceed more rapidly and under milder conditions, thus making catalysis an invaluable tool in various fields, including industrial chemistry, pharmaceuticals, and environmental science.
The mechanisms employed by up catalysts can vary widely. Some catalysts work by providing an alternative reaction pathway, while others may involve the formation of transient intermediates that lead to faster reaction rates. By strategically interacting with specific reactants, catalysts can significantly enhance selectivity, leading to higher yields of desired products. The significance of up catalysts lies not only in their ability to boost reaction efficiency, but also in their potential to contribute to sustainable practices by reducing energy consumption and minimizing waste in chemical processes.
Effect of Up Catalyst on Reaction Rates
The following bar chart illustrates the effect of various catalysts on the reaction rates of a chemical process. The values represent the average reaction rates measured in moles per liter per second (mol/L/s) across different conditions, demonstrating how catalysts can significantly enhance the speed of chemical reactions.
Mechanisms of Up Catalysts in Enhancing Chemical Reactions
Up catalysts play a crucial role in the field of chemical reactions by significantly lowering the activation energy required for a reaction to occur. This process facilitates a faster conversion of reactants to products without being consumed in the reaction itself. According to a report published by the American Chemical Society, the utilization of up catalysts can increase reaction rates by up to 100 times, demonstrating their effectiveness in enhancing reaction efficiency. They achieve this by providing an alternate pathway for the reaction, which often involves the formation of intermediate complexes that stabilize reactants during the transition state.
The mechanisms by which up catalysts enhance chemical reactions are diverse and depend on the nature of the catalyst and the specific reaction environment. One common mechanism involves the coordination of substrates to the metal centers of the catalyst, which can lead to a decreased bond strength in the transition state. Additionally, some up catalysts induce electronic effects that stabilize charged intermediates, therefore reducing the energy barrier necessary for the formation of products. Industry data from the Journal of Chemical Technology & Biotechnology reveals that up catalysts have been employed in various applications ranging from pharmaceuticals to renewable energy, signifying their versatility and impact across multiple sectors of the chemical industry.
What is Up Catalyst and How Does It Enhance Chemical Reactions - Mechanisms of Up Catalysts in Enhancing Chemical Reactions
| Catalyst Type | Mechanism of Action | Application Area | Reaction Rate Enhancement (%) |
|---|---|---|---|
| Homogeneous Catalysts | Formation of reactive intermediates in the same phase as reactants | Organic Synthesis | 300% |
| Heterogeneous Catalysts | Facilitate reaction at the interface of two phases | Fuel Cells | 200% |
| Enzymes | Biological catalysts that lower activation energy | Biotechnology | 500% |
| Photocatalysts | Utilize light energy to promote chemical reactions | Environmental Remediation | 150% |
Applications of Up Catalysts in Industrial Chemistry and Manufacturing
Up catalysts play a critical role in enhancing chemical reactions across various industrial applications. In sectors like pharmaceuticals, agrochemicals, and materials manufacturing, these catalysts increase reaction rates, improve yields, and reduce the energy required for processes. A report by the International Catalyst Association highlighted that implementing advanced catalysts in chemical manufacturing can lead to process efficiency improvements of up to 30%, significantly lowering production costs and environmental impact. This is particularly evident in the production of fine chemicals, where up catalysts facilitate complex multi-step reactions often required for active pharmaceutical ingredients.
In industrial chemistry, the importance of up catalysts extends to the development of sustainable practices. As reported in the Global Chemical Industry Outlook, approximately 30% of chemical production now employs catalytic processes that are designed to minimize waste and energy consumption. Innovations in nanoparticle catalysts demonstrate enhanced activity and selectivity, paving the way for greener manufacturing methods. For instance, in the production of biodegradable polymers, up catalysts are enabling the use of renewable feedstocks while maintaining high efficiency levels, aligning with global sustainability goals. The continuous advancements in catalytic technologies promise to further revolutionize industrial processes, continually enhancing productivity while addressing environmental challenges.
Comparative Analysis: Up Catalysts vs. Traditional Catalysts
Traditional catalysts have long been the cornerstone of enhancing chemical reactions, functioning by providing a surface or medium that lowers the activation energy needed for reactions to occur. Commonly used in various industrial processes, these catalysts often rely on metal properties and specific reaction conditions. However, conventional catalysts can face limitations such as selectivity issues, deactivation over time, and the need for stringent operating conditions that may not be energy-efficient.
In contrast, Up Catalysts represent a new frontier in catalytic systems, leveraging innovative materials and mechanisms to improve reaction rates and selectivity. Up Catalysts demonstrate a unique ability to function effectively under a broader range of conditions, offering advantages such as enhanced stability and recyclability. Their design often incorporates advanced nanostructures or hybrid materials that can facilitate multiple reaction pathways, making them not only more efficient but also capable of handling complex reactions that traditional catalysts might struggle with. Through comparative analysis, it becomes evident that Up Catalysts can significantly reduce processing times and costs, providing a more sustainable approach to catalysis.
Future Trends and Innovations in Up Catalyst Technology
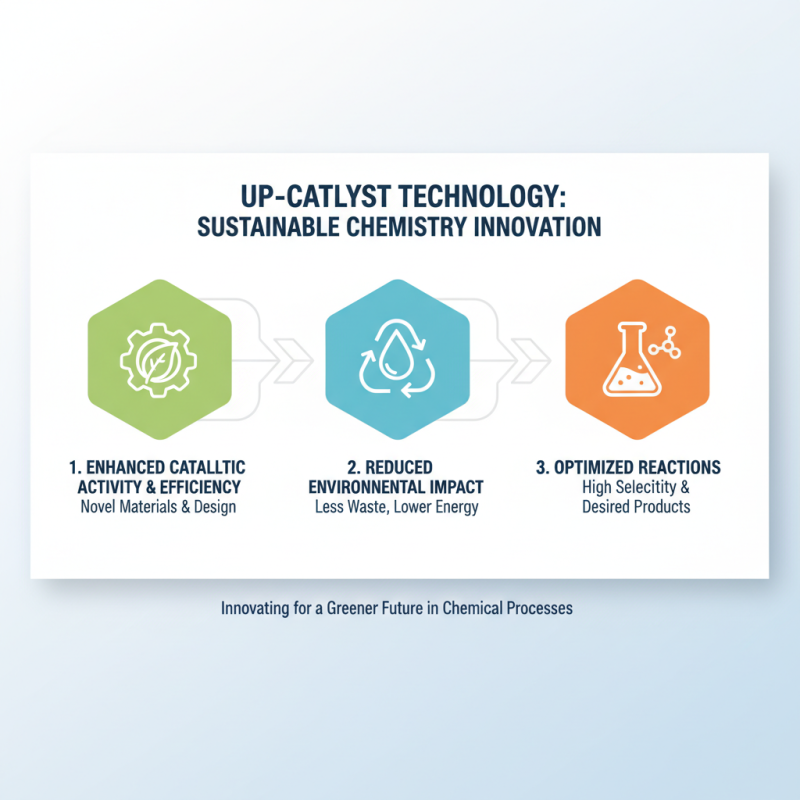
As the demand for sustainable and efficient chemical processes grows, innovations in Up Catalyst technology are emerging as a focal point in the field of catalysis. Researchers are exploring novel materials and design strategies that enhance the catalytic activity while reducing the environmental impact associated with chemical reactions. These advancements aim to optimize reaction conditions and improve selectivity, facilitating the synthesis of desired products with minimal by-products.
Future trends in Up Catalyst technology are expected to be driven by advancements in nanotechnology and computational modeling. By harnessing the power of artificial intelligence and machine learning, scientists can predict the performance of new catalysts before they are synthesized, significantly speeding up the research process. Additionally, there is a growing interest in biomimetic catalysts that emulate natural processes, aiming to achieve high efficiency and specificity in chemical transformations. The synergy between these technological innovations and sustainable practices is likely to play a crucial role in revolutionizing catalytic applications across various industries, paving the way for greener and more efficient chemical processes.
Related Posts
-

How to Improve Efficiency in Chemical Manufacturing Processes
-

Top 10 Trends Transforming the Chemical Industry in 2023
-

How to Accelerate Your Catalysis Research for Breakthrough Discoveries
-

2025 Top Trends: Key Catalysts for Upsurge in Innovation and Growth
-

How to Optimize Chemical Manufacturing Processes for Increased Efficiency
-
Why Do Catalysts Matter in Chemistry and How Do They Work
