Top 10 Types of Chemical Catalysts You Should Know?
In the realm of modern chemistry, understanding the role of a chemical catalyst is essential. Dr. Emily Carter, a leading expert in catalysis, states, “Catalysts are the unsung heroes of chemical reactions.” They speed up reactions without being consumed. This ability makes them crucial in various industries.
There are countless types of chemical catalysts, each serving distinct purposes in different reactions. Some are made of precious metals, while others are based on more abundant materials. This diversity allows chemists to optimize processes for cost, efficiency, and environmental impact.
However, not all catalysts function as expected. Inefficiencies can arise due to factors like temperature or pressure variations. These challenges remind us that the world of chemical catalysts is complex and not fully understood. Continuous research is vital to enhance our understanding and applications.
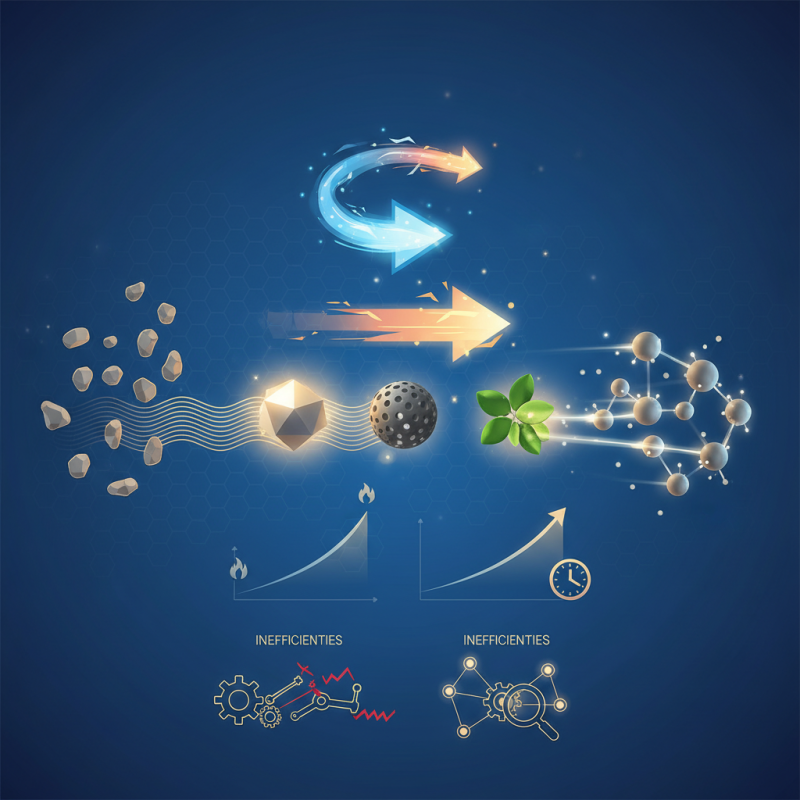
Types of Chemical Catalysts: An Overview
Chemical catalysts play a vital role in various chemical reactions. Their primary function is to accelerate reactions without being consumed in the process. This characteristic makes them essential in industries like pharmaceuticals, petrochemicals, and automotive manufacturing.
There are several types of catalysts. Homogeneous catalysts are in the same phase as the reactants. They often offer high reaction rates. However, they can be challenging to separate from products. Heterogeneous catalysts exist in a different phase. They are often more manageable but may have lower activity. Enzymes are a fascinating subset, acting as biological catalysts. They are highly specific and operate under mild conditions.
Each catalyst type has its benefits and drawbacks. Sometimes, the efficiency of a catalyst may diminish over time due to poisoning or deactivation. Choosing the right catalyst requires careful consideration. Not all reactions benefit from the same type. Experimentation is often needed to determine the best option. Understanding these nuances can change the way we approach reactions in chemistry.
Homogeneous Catalysts and Their Applications
Homogeneous catalysts are substances that exist in the same phase as the reactants. They often provide unique advantages in chemical reactions. These catalysts can increase reaction rates and improve yield. They are commonly used in industries such as pharmaceuticals and petrochemicals. The ability to easily manipulate conditions makes them highly versatile.
One popular application is in the production of fine chemicals. For instance, acidic homogeneous catalysts are crucial in esterification reactions. They can enhance selectivity, but there are challenges to consider. Over time, these catalysts may degrade. Additionally, separating them from products can be difficult. This often leads to environmental concerns and waste generation.
Despite their benefits, homogeneous catalysts aren't perfect. Sometimes, their effectiveness diminishes with repeated use. This can raise questions about sustainability. Researchers are continuously looking for strategies to address these issues. New methods aim to recover and reuse these catalysts more efficiently, which could lead to greener chemistry in the future.
Heterogeneous Catalysts: Features and Examples
Heterogeneous catalysts play a vital role in various chemical reactions. They exist in a different phase than the reactants, typically as solids in contact with liquids or gases. A key feature is their ability to increase reaction rates without being consumed. This attribute makes them highly valuable in industrial applications.
One prominent example of a heterogeneous catalyst is platinum in catalytic converters. This catalyst helps convert harmful gases into less harmful emissions. While effective, the use of precious metals raises concerns about cost and resource availability. Not all reactions can utilize these catalysts efficiently; sometimes they lead to unanticipated side reactions.
Another common example is zeolites in petroleum refining. They serve as molecular sieves, selectively absorbing compounds. However, their effectiveness can diminish over time due to coking, where carbon builds up and clogs the catalyst surface. It's crucial to monitor and maintain these catalysts to sustain their performance. Understanding the limitations of heterogeneous catalysts can lead to better innovations in the field.
Top 10 Types of Chemical Catalysts You Should Know
| Catalyst Type | Features | Examples |
|---|---|---|
| Heterogeneous Catalysts | Catalysts that exist in a different phase than the reactants. | Iron in Haber process, Nickel in hydrogenation |
| Homogeneous Catalysts | Catalysts that exist in the same phase as the reactants. | Acids in esterification reactions |
| Enzymatic Catalysts | Biological catalysts that are usually proteins. | Amylase, Lipase, Catalase |
| Metallic Catalysts | Metals used to accelerate reactions, often in fine powder form. | Platinum, Palladium |
| Acid-Base Catalysts | Catalysts that donate or accept protons in reactions. | Sulfuric acid, Sodium hydroxide |
| Biocatalysts | Catalysts derived from biological sources. | Glucose oxidase, Chymotrypsin |
| Supported Catalysts | Catalysts that are dispersed on a support material to enhance activity. | Zeolites, Silica gel |
| Transition Metal Catalysts | Catalysts comprised of transition metals, often complexed with ligands. | Rhodium in hydroformylation |
| Organocatalysts | Small organic molecules used as catalysts. | Proline, Amino acids |
Enzymatic Catalysts and Their Role in Biochemical Reactions

Enzymatic catalysts play a crucial role in biochemical reactions. They are proteins that accelerate chemical reactions without being consumed. According to recent studies, enzymes can increase reaction rates by a factor of a thousand to a million. This capability makes them indispensable in biological systems.
Enzymes are highly specific. They target only certain substrates, ensuring precise outcomes in metabolic pathways. However, this specificity can be a double-edged sword. It limits the range of reactions they can catalyze. For instance, some enzymes require optimal pH and temperature to function effectively. Outside these parameters, their activity can decline sharply.
The efficiency of enzymatic catalysts is impressive, yet they are not perfect. Factors like enzyme concentration and inhibition can impact their performance. For example, a report from the Journal of Biological Chemistry highlighted that competitive inhibitors could reduce enzyme activity by up to 90%. This underscores the complexity of enzymatic reactions. Understanding these limitations is essential for optimizing their use in industrial and therapeutic applications.
Applications of Catalysts in Industrial Processes and Production
Catalysts play a vital role in industrial processes, driving chemical reactions efficiently. They help reduce energy consumption and waste. According to a 2022 report by the International Energy Agency, about 20% of global emissions reduction comes from using catalysts in industrial applications. This highlights their significance in tackling environmental challenges.
In petrochemical production, catalysts facilitate the conversion of raw materials into valuable products. For instance, the use of zeolite catalysts helps in refining crude oil. This process improves yield and product quality. Additionally, in the production of ammonia, iron-based catalysts are crucial. They enable the Haber-Bosch process, which accounts for around 2% of global energy consumption. This fact raises concerns about energy efficiency in large-scale operations.
Despite their advantages, relying on catalysts can have drawbacks. The stability and lifespan of catalysts can vary, leading to process inefficiencies. For example, some catalysts may require frequent replacement, which adds to operational costs. Moreover, catalyst deactivation can occur due to poisoning or fouling. This unpredictability can disrupt production timelines and increase waste. While catalysts are indispensable, they also require careful management and optimization to maximize their benefits.
Related Posts
-
What is a Catalyst in Catalysis? Understanding Their Role and Impact in Chemical Reactions
-
Top 10 Catalytic Reactions You Should Know For Chemistry Success
-
Why Do Catalysts Matter in Chemistry and How Do They Work
-

How to Accelerate Your Catalysis Research for Breakthrough Discoveries
-

How to Leverage Catalyst Technologies for Business Growth and Innovation
-

What Are the 4 Types of Catalysts and Their Functions in Chemistry?
