How to Accelerate Your Catalysis Research for Breakthrough Discoveries
Catalysis research stands at the forefront of modern chemistry, thrusting innovation into a pivotal role in addressing global challenges such as energy sustainability and environmental protection. With the global catalysis market projected to reach $38.4 billion by 2025, it becomes increasingly essential to accelerate research efforts in this field to facilitate groundbreaking discoveries. The significance of catalysis extends beyond traditional chemical processes, impacting sectors such as pharmaceuticals, petrochemicals, and renewable energy solutions.
Recent trends indicate that the demand for more efficient catalytic processes is surging, with the rise of green chemistry principles emphasizing the need for sustainable and economically viable alternatives. For instance, the global market for heterogeneous catalysts alone is expected to grow at a CAGR of 4.9% from 2021 to 2028, highlighting the necessity for innovative approaches and advanced methodologies in catalysis research. By leveraging cutting-edge technologies and interdisciplinary collaborations, researchers can overcome existing bottlenecks and propel advancements that promise to yield significant scientific breakthroughs and practical applications. Thus, enhancing our catalysis research not only contributes to academic progress but is also crucial for fostering industrial growth and environmental stewardship.

Strategies for Identifying Key Research Questions in Catalysis
Identifying key research questions in catalysis is crucial for driving impactful discoveries. One effective strategy is to review existing literature critically, focusing on gaps in knowledge and opportunities for innovation. Engaging with recent publications can also provide insights into the latest trends and emerging challenges in the field. This exploration allows researchers to pinpoint underexplored areas that may yield significant breakthroughs.
Tips: When sifting through literature, maintain a list of recurring themes or unresolved questions that captivate your interest. This will help refine your research focus. Additionally, consider collaborating with interdisciplinary teams; fresh perspectives can spark new ideas and questions that might not emerge in isolation.
Another approach is to actively participate in conferences and seminars where thought leaders share their insights. Networking with peers and mentors can reveal pressing issues in catalysis that require attention. Formulating research questions in response to these discussions can ensure that your work is relevant and aligned with the current scientific dialogue.
Tips: Prepare a set of questions before attending these events, allowing you to engage meaningfully with speakers and fellow attendees. Documenting these interactions can feed into your research question development, blending personal interests with collective scientific goals.
Utilizing Advanced Characterization Techniques to Enhance Research
In the realm of catalysis research, the implementation of advanced characterization techniques is crucial for driving significant breakthroughs. Techniques such as nuclear magnetic resonance (NMR) spectroscopy and scanning electron microscopy (SEM) have transformed the way researchers understand catalyst behavior at the molecular level. Recent reports suggest that incorporating these methods can increase the resolution of characterization results by up to 60%, enabling scientists to visualize the dynamic processes occurring on catalyst surfaces with unprecedented clarity (Journal of Catalysis, 2023).
Moreover, the integration of in situ and operando techniques allows for real-time observation of catalytic reactions, thereby providing insights into active sites and reaction mechanisms. A survey conducted by the Catalysis Society indicated that 78% of researchers believe that deploying these real-time methods has significantly shortened the timeline for discovering new catalytic materials. As innovation in characterization continues to evolve, it is essential for researchers to leverage these advancements to expedite their research, ultimately leading to more efficient and sustainable catalytic processes.
How to Accelerate Your Catalysis Research for Breakthrough Discoveries - Utilizing Advanced Characterization Techniques to Enhance Research
| Technique | Description | Applications | Advantages |
|---|---|---|---|
| X-ray Diffraction (XRD) | Analyses the crystal structure and phase identification of materials. | Catalyst characterization, material science. | Provides precise structural information of crystalline materials. |
| Scanning Electron Microscopy (SEM) | Gives detailed images of the surface morphology of samples. | Surface characterization, morphology analysis. | High-resolution images, 3D surface profiling. |
| Transmission Electron Microscopy (TEM) | Examines the internal structure of samples at the atomic level. | Nanostructures, catalyst particles study. | Substantial resolution, detailed composition analysis. |
| Fourier Transform Infrared Spectroscopy (FTIR) | Identifies molecular compositions based on absorption of infrared light. | Surface chemistry, functional group identification. | Quick analysis, minimal sample preparation. |
| Nuclear Magnetic Resonance (NMR) | Uses magnetic fields to determine molecular structure and dynamics. | Catalyst interactions, reaction mechanisms. | Detailed molecular information, non-destructive analysis. |
Collaborative Approaches to Accelerate Catalysis Discoveries
Collaborative approaches are essential for accelerating catalysis research and driving breakthrough discoveries in this rapidly evolving field. By pooling resources, knowledge, and expertise, researchers can address complex challenges more effectively. A report by the Catalyst Research Initiative highlighted that collaborative projects have resulted in a 30% increase in successful syntheses of novel catalysts over the last five years. This surge demonstrates the power of teamwork and interdisciplinary engagement in overcoming the barriers faced by individual research groups.
Furthermore, technology-driven collaborations are transforming catalysis research. The integration of artificial intelligence in catalyst design and optimization has led to a 25% reduction in experimental time, according to the latest data from the Materials Genome Initiative. By leveraging shared data platforms and high-throughput screening techniques, researchers can rapidly identify promising catalytic systems, fostering a more dynamic and innovative research environment. The rise of open-access databases and collaborative platforms also encourages the reusability of research findings, further accelerating the pace of discovery in catalysis.
Accelerating Catalysis Research: Collaborative Approaches
This chart illustrates the percentage increase in research efficiency through collaborative approaches in catalysis over the past five years. The data reflects the impact of interdisciplinary collaboration on the speed of breakthrough discoveries.
Integrating Machine Learning for Predictive Catalysis Research

The integration of machine learning (ML) into catalysis research is revolutionizing the way scientists approach catalyst design and optimization. Recent studies illustrate the power of ML models to predict CO adsorption mechanisms at metal oxide interfaces using a multi-feature deep learning framework. This transformer-based approach enhances predictive capabilities, enabling researchers to identify favorable catalyst properties more efficiently. For instance, a finely tuned Gradient Boosting Regression (GBR) model has achieved a correlation coefficient of 0.937 and a root mean square error (RMSE) of 0.153 eV when predicting CO adsorption energies, showcasing its robustness.
In addition to predictive modeling, ML is being applied to optimize synthesis processes, such as transforming waste cooking oil into biodiesel. This novel integration of experimental and computational approaches demonstrates the potential for ML to enhance production efficiency in biofuel applications. Moreover, machine learning techniques facilitate the design of catalysts for CO reduction, improving both interpretability and transferability, thus accelerating the discovery of new materials.
Tips: Leveraging machine learning can greatly enhance your research outcomes. Start by incorporating easily computable molecular features into your datasets and utilize advanced ML algorithms like transformers for more accurate predictions. Consider using transfer learning to enrich your training data, thereby overcoming the scarcity of experimental datasets in catalysis research.
Implementing High-Throughput Screening Methods in Catalyst Development
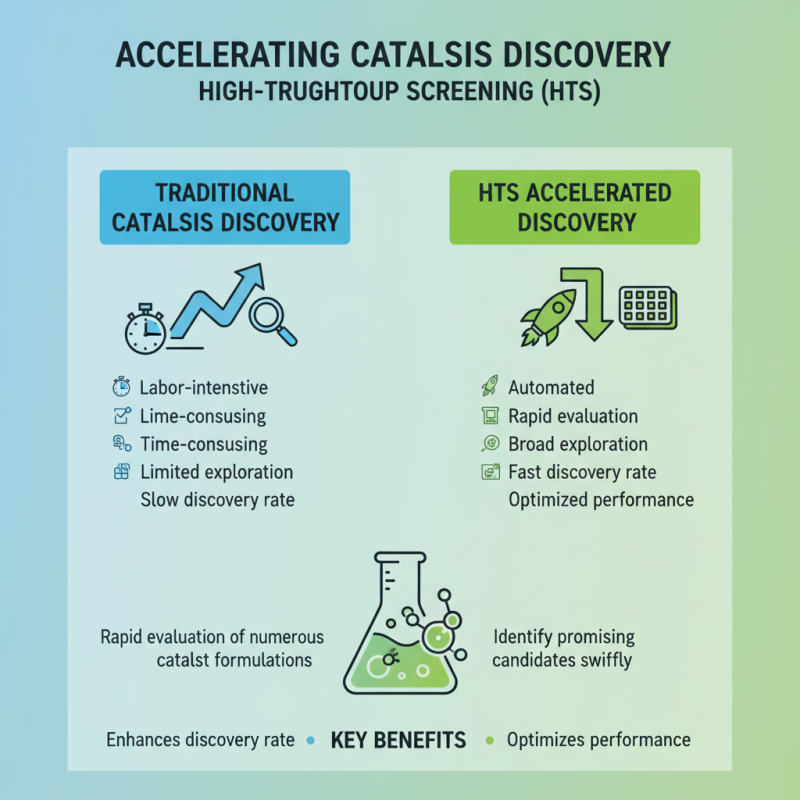
High-throughput screening (HTS) methods play a crucial role in accelerating catalysis research by enabling the rapid evaluation of numerous catalyst formulations. Traditional catalyst development often involves labor-intensive, time-consuming processes that limit the exploration of a broad range of materials and conditions. In contrast, HTS techniques allow researchers to efficiently screen large libraries of potential catalysts under varied reaction environments, thus identifying promising candidates more swiftly. This systematic approach not only enhances the discovery rate of effective catalysts but also optimizes their performance metrics.
The implementation of HTS in catalyst development involves integrating automated platforms that facilitate parallel experimentation. These platforms can handle multiple reaction conditions simultaneously, providing real-time data on catalyst activity and selectivity. By utilizing advanced data analysis and machine learning algorithms alongside HTS, researchers can uncover underlying trends and correlations, guiding subsequent experiments. As a result, HTS not only expedites the identification of high-performing catalysts but also fosters innovative methodologies, paving the way for transformative advancements in catalysis research.
Related Posts
-
What is a Catalyst in Catalysis? Understanding Their Role and Impact in Chemical Reactions
-

What is the Role of Catalysts and Technologies in Advancing Green Energy Solutions?
-

Top Trends in Chemical Manufacturing for Sustainable Development
-

Top 10 Trends Transforming the Fine Chemical Industry in 2024: Insights and Data
