What Are the 4 Types of Catalysts and Their Functions in Chemistry?
In the world of chemistry, catalysts play a vital role in facilitating reactions. The "4 catalysts" are essential for understanding various processes. According to a 2022 industry report from ChemInsights, catalysts can increase reaction rates by up to 1,000 times. This efficiency is crucial for industrial applications.
Dr. Emily Thompson, a leading expert in catalysis, stated, "The right catalyst transforms not just reactions, but entire industries." She emphasizes the significance of understanding how different catalysts function. The four types of catalysts — homogeneous, heterogeneous, enzyme-based, and photocatalysts — each serve distinct purposes.
As the industry evolves, the demand for effective catalysts rises. Challenges persist in optimizing their use. Not every reaction benefits from a catalyst, and sometimes the choice impacts yield. Thus, awareness of the "4 catalysts" is not just academic; it's essential for practical application. The future of chemical innovation hinges on refining these tools for better outcomes.

Types of Catalysts and Their Role in Chemical Reactions
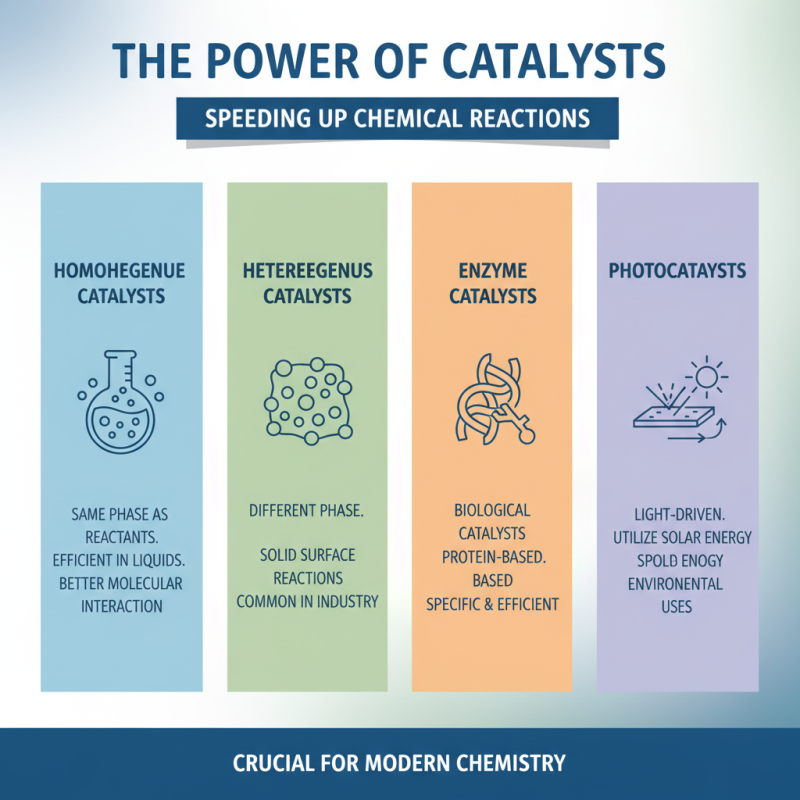
Catalysts play a crucial role in chemical reactions. They speed up reactions without being consumed. There are four main types of catalysts: homogeneous, heterogeneous, enzyme, and photocatalysts. Each type has unique functions. Homogeneous catalysts exist in the same phase as the reactants. This makes them particularly effective in liquid reactions. They allow for better interaction between molecules.
Heterogeneous catalysts, however, are in a different phase than the reactants. They often take the form of solids in gas or liquid reactions. These catalysts provide surfaces for reactions to occur efficiently. Enzymes are biological catalysts. They are highly specific and sensitive to conditions. Their activity can change with temperature and pH. Photocatalysts utilize light to drive chemical reactions. They can be quite effective in environmental applications.
The effectiveness of catalysts often depends on various factors. Temperature, concentration, and surface area can significantly influence their performance. Sometimes, catalysts may degrade over time. This can lead to decreased efficiency. Understanding these nuances is vital. Recognizing these limitations can guide future research and applications in chemistry. Optimizing catalyst use remains an ongoing challenge for scientists.
Homogeneous Catalysts: Definition and Examples
Homogeneous catalysts play a significant role in chemical reactions. These catalysts exist in the same phase as the reactants. This uniformity allows for better interaction. Homogeneous catalysts are often found in solutions, making them highly effective. They enhance reaction rates without undergoing permanent changes themselves.
Common examples include acid-base catalysts. For instance, sulfuric acid can speed up the esterification process. In this context, the acid donates protons, facilitating the reaction. Another example is transition metal complexes. These complexes catalyze reactions like hydrogenation. They adjust the rates of these reactions by forming temporary bonds with reactants.
While homogeneous catalysts are efficient, they have their limitations. They can be challenging to separate from products. This separation issue can lead to additional costs. Sometimes, reactions may produce unwanted side products. These could complicate purification processes, making industrial applications tricky.
What Are the 4 Types of Catalysts and Their Functions in Chemistry?
| Type of Catalyst | Definition | Examples | Functions |
|---|---|---|---|
| Homogeneous Catalysts | Catalysts that exist in the same phase as the reactants | Acids and Bases in esterification | Increase reaction rates, provide alternative pathways |
| Heterogeneous Catalysts | Catalysts that are in a different phase from the reactants | Platinum in hydrogenation reactions | Facilitate reactions on their surface, allowing easier access |
| Biocatalysts | Catalysts that are made of enzymes and proteins | Amylase in starch breakdown | Increase reaction specificity and lower energy barriers |
| Organocatalysts | Small organic molecules used as catalysts | Proline in asymmetric synthesis | Facilitate reactions without metals, often more environmentally friendly |
Heterogeneous Catalysts: Structure and Mechanism

Heterogeneous catalysts play a crucial role in various chemical reactions. They exist in a different phase than the reactants. This phase difference enhances the interaction between the catalyst and the reactants. For instance, solid catalysts can promote reactions in gas or liquid phases. Their surfaces host active sites where reactions take place. A report indicates that about 70% of industrial chemicals use heterogeneous catalysts.
The mechanism of heterogeneous catalysis involves adsorption, reaction, and desorption stages. Molecules adhere to the catalyst surface, forming a temporary bond. This interaction lowers the activation energy needed for the reaction. Furthermore, research shows that the specific surface area of the catalyst significantly affects its efficiency. A higher surface area usually leads to increased reaction rates. Yet, achieving the optimal structure is challenging. It often requires balancing activity and selectivity.
Developing better heterogeneous catalysts remains a complex task. Many existing catalysts suffer from issues like deactivation due to poisoning or sintering. This limits their lifespan and efficiency. Future research focuses on creating more robust catalysts. Investigating materials at the nanoscale has shown promising results, with improvements in both performance and stability. However, there's still much to be done to understand these processes fully.
Biocatalysts: Nature's Catalysts in Biological Processes
Biocatalysts are essential to life. These natural catalysts drive biochemical reactions in living organisms. Enzymes, a type of biocatalyst, help break down food in our digestive systems. Each enzyme works on specific substrates. For example, amylase targets starch, transforming it into simple sugars. This process is crucial for energy production.
Biocatalysts are highly efficient. They accelerate reactions at lower temperatures than chemical catalysts. This is why life can thrive in various environments. Nature has perfected these catalysts over billions of years. However, they are not infallible. Sometimes, enzymes malfunction, leading to metabolic disorders. Scientists study these failures to understand life better.
Biocatalysis also finds its place in industry. It aids in sustainable practices, reducing environmental impact. Reactions that once required harsh chemicals now use gentle enzymes. Nevertheless, scaling up these processes is challenging. Stability and efficiency can diminish outside the biological environment. Achieving optimal conditions for biocatalysts remains an area for further exploration.
Physical Catalysts: Surface Effects and Their Applications
Physical catalysts play a vital role in chemical reactions by enhancing the efficiency of surface interactions. Their primary function revolves around modifying reaction pathways. This modification can lead to lower activation energy and increased reaction rates. According to a report from the American Chemical Society, surface catalysts can boost reaction speeds by up to 200%. This can drastically reduce energy consumption in industrial processes.
Specific applications highlight the importance of physical catalysts. For instance, heterogeneous catalysts are widely used in the petrochemical industry for refining processes. Their surface properties dictate how reactants adsorb and transform on their surfaces. A study found that the right catalytic surface can increase yield by nearly 30%. However, the challenge remains in optimizing these surfaces for maximum efficiency.
Research indicates that not all surfaces perform equally. Some catalysts can become deactivated over time due to fouling or sintering. This can lead to significant downtime and economic losses. For many industries, refining the surfaces of catalysts is an ongoing task. It requires a balance between cost, effectiveness, and longevity. There's much to learn and improve in this field.
Types of Catalysts and Their Functions in Chemistry
This chart illustrates the different types of catalysts and their functions in chemistry. The data represents the effectiveness of each catalyst type based on their general applications in various chemical reactions.
Related Posts
-

How to Accelerate Your Catalysis Research for Breakthrough Discoveries
-
What is a Catalyst in Catalysis? Understanding Their Role and Impact in Chemical Reactions
-

What is the Role of Catalysts and Technologies in Advancing Green Energy Solutions?
-

How to Leverage Catalyst Technologies for Business Growth and Innovation
-
Top 10 Catalytic Reactions You Should Know For Chemistry Success
-

How to Leverage Catalyst Services for Business Growth and Innovation in 2025
